Difference between revisions of "Main Page"
(→Computational models of human memory) |
|||
| (165 intermediate revisions by 8 users not shown) | |||
| Line 11: | Line 11: | ||
|- | |- | ||
| [[File:Models-thumb.png|frameless|left|border|150px|link=#Computational models of human memory]] | | [[File:Models-thumb.png|frameless|left|border|150px|link=#Computational models of human memory]] | ||
| − | | width=" | + | |- |
| + | | width="320pt" | <span style="font-size: 15pt; line-height: 130%">[[#Computational models of human memory|Computational models of human memory]]</span> | ||
|} | |} | ||
| | | | ||
| Line 17: | Line 18: | ||
|- | |- | ||
| [[File:Ecog-thumb.png|frameless|left|border|150px|link=#Neural oscillatory correlates of episodic memory]] | | [[File:Ecog-thumb.png|frameless|left|border|150px|link=#Neural oscillatory correlates of episodic memory]] | ||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | | width="320pt" | <span style="font-size: 15pt; line-height: 130%">[[#Neural oscillatory correlates of episodic memory|Neural oscillatory correlates of episodic memory]]</span> |
| + | |} | ||
| + | | | ||
{| border="0" cellpadding="5" style="border: 1px solid darkgray;" | {| border="0" cellpadding="5" style="border: 1px solid darkgray;" | ||
|- | |- | ||
| − | | [[File: | + | | [[File:MillerThumb.png|frameless|left|border|150px|link=#Human spatial memory and cognition]] |
| − | | width=" | + | |- |
| + | | width="320pt" | <span style="font-size: 15pt; line-height: 130%">[[#Human spatial memory and cognition|Human spatial memory and cognition]]</span> | ||
|} | |} | ||
| | | | ||
{| border="0" cellpadding="5" style="border: 1px solid darkgray;" | {| border="0" cellpadding="5" style="border: 1px solid darkgray;" | ||
|- | |- | ||
| − | | [[File: | + | | [[File:closed_loop_thumbnail.png|frameless|left|border|150px|link=#Cognitive Neuromodulation]] |
| − | | width=" | + | |- |
| + | | width="320pt" | <span style="font-size: 15pt; line-height: 195%">[[#Cognitive Neuromodulation|Cognitive Neuromodulation]]</span> | ||
|} | |} | ||
| + | | | ||
| + | <!-- |- | ||
| + | | colspan="4" align="center" style="font-style: bold; font-size: 15pt;" | New: Find a collection of [[Press|news articles about the CML's research here]]--> | ||
|} | |} | ||
| Line 37: | Line 43: | ||
{| cellpadding="20" style="margin: 0 auto;" | {| cellpadding="20" style="margin: 0 auto;" | ||
| − | | width=" | + | | width="1200pt" | <span style="font-size: 14pt; line-height: 130%"> |
| + | Our lab investigates human memory and its neural basis using a combination of behavioral, computational, and neurophysiological methods. In our computational investigations, we build mathematical and computer-simulation models to account for the dynamics of memory retrieval in a variety of episodic and spatial memory tasks. Because behavioral data provides a sparse reflection of the brain’s activity supporting memory, we simultaneously record neurophysiological signals as patients with arrays of implanted electrodes perform memory tasks. In these investigations we study neural activity at multiple spatial scales, ranging from individual neurons to spatially-distributed networks of field-potential activity supporting memory. Several of our current projects also use electrical stimulation to manipulate memory circuits, both for understanding basic memory mechanisms and also for developing therapies to restore memory in patients with brain injury or neurological disease. | ||
| + | |||
| + | |||
| + | |||
| + | | | ||
| + | {| cellpadding="20" style="border: 1px solid darkgray;" | ||
| width="100pt" style="background-color:#dddddd;"| [[File:fhm_cover.png|175px|link=Foundations of Human Memory]] | | width="100pt" style="background-color:#dddddd;"| [[File:fhm_cover.png|175px|link=Foundations of Human Memory]] | ||
| − | <big>[[Foundations of Human Memory]]</big><br />by Michael J. Kahana<br /><br />Please [[Foundations of Human Memory|click here]] for more information and errata. | + | <big>[[Foundations of Human Memory]]</big><br />by [[Michael J. Kahana]]<br /><br />Please [[Foundations of Human Memory|click here]] for more information and errata. |
| + | |} | ||
|} | |} | ||
== Computational models of human memory == | == Computational models of human memory == | ||
| − | To explain the processes underlying encoding, organization and retrieval of episodic memories, | + | To help explain the processes underlying encoding, organization and retrieval of episodic memories, we have developed, extended and refined a class of models based on the idea that items in memory become associated with a time-varying representation of spatio-temporal context. The temporal context model (TCM; [[Publications#HowaKaha02|Howard and Kahana, 2002]] and TCM-A [[Publications#SedeEtal08|Sederberg, Howard, and Kahana, 2008]]) sought to explain the time-scale invariance of recency and contiguity effects in free recall, and dissociations between recall of recent and remote memories. Subsequent modeling work generalized TCM beyond temporal context to account for the influence of semantic knowledge on recall dynamics (CMR, [[Publications#PolyEtal09|Polyn, Norman, and Kahana (2009)]]). MATLAB scripts to run the CMR model [[CMR|can be downloaded here]]. |
| − | + | [[Publications#LohnEtal14| Lohnas, Polyn, and Kahana (2015)]] provided a major overhaul of the earlier CMR model, going beyond earlier modeling of individual lists to explain the interaction between memories learned across multiple experiences . In their CMR2 model, memory accumulates across multiple experimental lists, and temporal context is used both to focus retrieval on a target list and to censor retrieved information when its match to the current context indicates that it was learned in a non-target list. The model simultaneously accounts for a wide range of intralist and interlist phenomena, including the pattern of prior-list intrusions observed in free recall, build-up of and release from proactive interference, and the ability to selectively target retrieval of items on specific prior lists (Jang & Huber, 2008; Shiffrin, 1970). [[Publications#HealKaha15|Healey and Kahana (2015)]] used CMR2 to better understand why memory tends to get worse as we age. By fitting CMR2 to the performance of individual younger and older adults, they identified deficits in four critical processes: sustaining attention across a study episode, generating retrieval cues, resolving competition, and screening for inaccurate memories (intrusions). Healey and Kahana also extended CMR2 to simulate a recognition memory task using the same mechanisms the free recall model uses to reject intrusions. Without fitting any additional parameters, the model accounts for age differences in recognition memory accuracy. Confirming a prediction of the model, free recall intrusion rates correlate positively with recognition false alarm rates. MATLAB scripts to run the CMR2 model [[Publications#LohnEtal14|can be downloaded here]]. Cohen and Kahana (2021, ''Psychological Review'') introduced CMR3 to include the critical role of arousal and emotion in human memory. They applied their model to diverse phenomena including the role of emotion in organizing memories, state-dependent and mood congruent memory, the role of emotional experiences in producing persistent mood states, including depression, and a novel account of PTSD and its treatment. | |
| − | + | ||
| − | + | A review of this line of research appeared in Kahana (2020), Computational Models of Memory Search, in the ''Annual Review of Psychology''. '''Python code that runs CMR2 and CMR3 may be downloaded from the lab's publication page.''' | |
| − | + | ||
| − | |||
| − | |||
{| style="margin: 0 auto;" cellpadding="20" | {| style="margin: 0 auto;" cellpadding="20" | ||
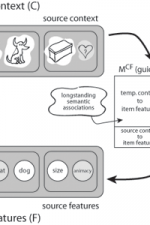
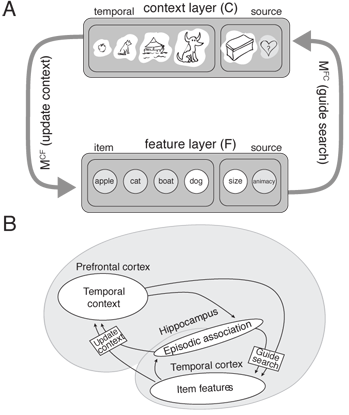
| − | | [[File: | + | |[[File:cmr_neuro.png|none|thumb|400px|''Fig. 1:'' '''The context-maintenance and retrieval model.''' A. Schematic of CMR. When an item is studied its feature representation (fi) is activated on F. The feature representation contains both item and source features (e.g., size / animacy judgment). An associative weight matrix (MFC) allows each item to update a context representation that contains the item and source features of recently studied items. During study, the features of each item are associated with coactive context elements. During recall, the context representation reactivates (through MCF) the features of recently studied items. B. Hypothesized interactions between prefrontal cortex, temporal cortex, and medial temporal lobe during memory encoding and retrieval predicted by CMR.]] |
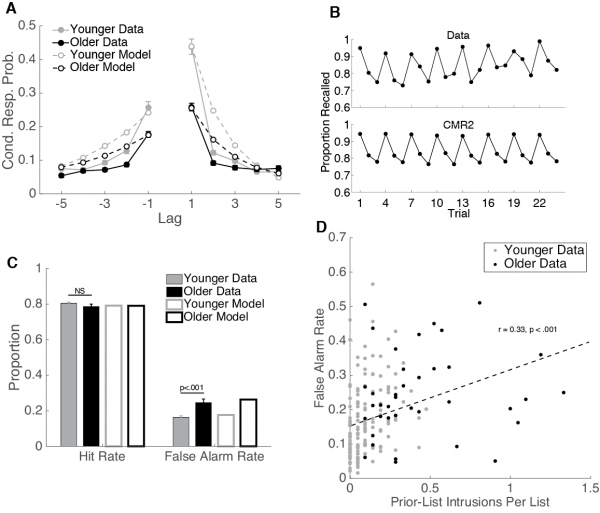
| − | |[[File: | + | |[[File:cmr2_data.png|center|thumb|600px|''click to enlarge''<br /><br />''Fig. 2:'' '''Data and CMR2 Simulations.''' A. This curve shows the probability of making a recall to serial position i+lag immediately following recall of serial position ''i''—that is, the conditional-response probability (CRP) as a function of lag. CMR2 captures older adults' temporal contiguity deficit. B. Release from proactive interference (PI). Participants studied lists of three items, each drawn from the same semantic category. After three lists from the same semantic category, subjects were then presented with a new set of three lists from a different semantic category. Top: Data from Loess (1967), note the scalloped pattern: performance declines across same-category lists and improves when a new category is presented. Bottom: CMR2 simulations capture the release from PI pattern. C. CMR2 simulations of hit and false alarm rates in recognition. D. As predicted by CMR2, recognition false alarm rates correlate with free recall intrusion rates. ]] |
|} | |} | ||
| − | + | == Neural and oscillatory correlates of episodic memory == | |
| − | {| style="margin: | + | We investigate the neurophysiology of episodic memory with electrocorticographic (ECoG) and single neuron recordings from neurosurgical patients who have had electrodes surgically implanted on the cortical surface of the brain or in the medial temporal lobes (including hippocampus) as part of the clinical process of localizing seizure foci. One focus of this research is to determine the oscillatory correlates of successful episodic memory formation and retrieval. Analyses of such recordings have shown that high-frequency activity (HFA, 70-150 Hz) increase while participants are studying words that they will successfully, as opposed to unsuccessfully, recall. HFA also rises just prior to successful vocal free recall (reviewed in [[Publications#BurkEtal15|Burke et al., 2015]]; for a video of the encoding effects click here [[HFA_Encoding| here]] ) for a video of the retrieval effect click here [[HFA_Retrieval| here]] ). |
| − | | | + | |
| + | {| style="margin: auto; width: 560px" cellpadding="20"; class="wikitable" | ||
| + | |<HTML5video width="560" height="315" autoplay="true" loop="true">FR</HTML5video> | ||
| + | <span style="font-size:90%"> ''Fig. 3:'' '''Free Recall Paradigm''' By using a free recall task in which participants study a list of words, we can measure episodic memory formation by comparing the spectral correlates associated with encoding items that are later recalled (red) or forgotten (blue). We record electroencephalographic (EEG) signals from subdurally implanted electrodes in patients with medically intractable epilepsy. We can extract spectral signals (power of a given frequency) from the raw EEG voltage traces for each item and measure when and where power at particular frequencies changes. Successful memory formation is associated with increases in HFA in left lateral temporal lobe, medial temporal lobe, and left prefrontal cortex. The same analyses can be performed on items during recall to assess when and where memories are retrieved. Successful memory retrieval is associated with increases in gamma band activity in the left neocortex and hippocampus as well as increases in theta band (4 -8 Hz) activity in right temporal lobe. ''' </span> | ||
|} | |} | ||
| − | + | The ability to reinstate this contextual information during memory search has been considered a hallmark of episodic, or event-based, memory. In [[Publications#MannEtal11a|Manning et al., 2011]], we sought to determine whether contextual reinstatement may be observed in electrical signals recorded from the human brain during episodic recall. We examined ECoG activity from 69 neurosurgical patients as they studied and recalled lists of words in a delayed free recall paradigm (Fig. 4A), and computed similarity between the ECoG patterns recorded just prior to each recall with those recorded after the patient had studied each word. We found that, upon recalling a studied word, the recorded patterns of brain activity were not only similar to the patterns observed when the word was studied, but were also similar to the patterns observed during study of neighboring list words, with similarity decreasing reliably with positional distance (Fig. 4C), just as predicted by context reinstatement models of free recall. The degree to which individual patients exhibited this neural signature of contextual reinstatement was correlated with the contiguity effect as seen in Fig. 4D. In this way, the study provides neural evidence for contextual reinstatement in humans. | |
| − | + | ||
| − | + | ||
{| style="margin: 0 auto;" cellpadding="20" | {| style="margin: 0 auto;" cellpadding="20" | ||
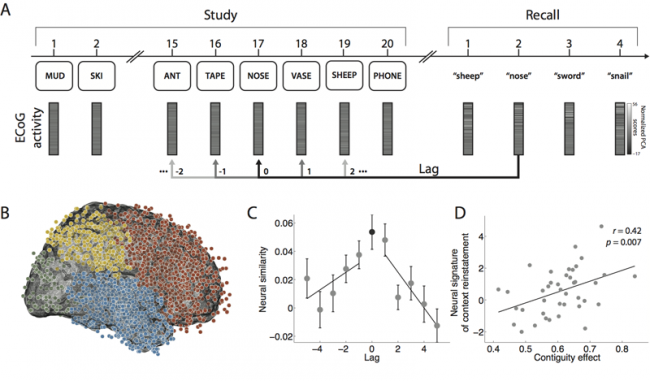
| − | | [[File: | + | |[[File:neuralContext.png|center|thumb|650px|''Fig. 4:'' '''Neural evidence for contextual reinstatement in humans.''' A. After studying a list of 20 words and performing a brief distraction task, a participant recalls as many words as he can remember, in any order. ECoG activity is recorded during each study and recall event. The similarity between the recorded patterns is computed as a function of lag. B. Each dot marks the location of a single electrode [temporal lobe (1,815 electrodes), frontal lobe (1,737 electrodes), parietal lobe (512 electrodes), and occipital lobe (138 electrodes)]. C. Similarity between the activity recorded during recall of a word from serial position ''i'' and study of a word from serial position ''i'' + lag. (Black dot denotes study and recall of the same word, i.e., lag = 0.) D. Participants exhibiting stronger neural signatures of context reinstatement also exhibited more pronounced contiguity effects (as measured by a percentile-based temporal clustering score). (Figure after Manning et al., 2011.)]] |
|} | |} | ||
== Human spatial memory and cognition == | == Human spatial memory and cognition == | ||
| − | Our lab is also interested in the neural mechanisms underlying human spatial cognition. In this work, we use virtual reality computer games | + | |
| + | |||
| + | Our lab is also interested in the neural mechanisms underlying human spatial cognition. In this work, we use virtual reality computer games in which participants learn the locations of landmarks in virtual environments. To download a sample of a YellowCab session, click [http://memory.psych.upenn.edu/files/misc/yc2_movie.mov here]. Using this approach, we have documented the existence and character of the 4-8 Hz theta rhythm in the human brain as participants learned to navigate through complex virtual environments ([[Publications#KahaEtal99b|Kahana et al., 1999]]; [[Publications#CaplEtal01|Caplan et al., 2001]]; [[Publications#CaplEtal03|Caplan et al., 2003]]; [[Publications#EkstEtal05|Ekstrom et al., 2005]]; [[Publications#JacoEtal09|Jacobs et al., 2010a]]). Recording individual neurons during virtual navigation, we have discovered "place cells" in the human brain. These cells, which are found primarily in the human hippocampus, become active when a given spatial location is being traversed ([[Publications#EkstEtal03|Ekstrom et al., 2003]]). We also identified several other cellular responses during navigation: cells that become active in response to viewing a salient landmark (from any location), cells that become active when searching for a particular goal location (irrespective of location or view), and cells that respond when traveling in a given direction (bearing/heading). | ||
[[Publications#JacoEtal10|Jacobs et al. (2010b)]] examined recordings of single-neuron activity from neurosurgical patients playing a virtual-navigation video game. In addition to place cells, which encode the current virtual location, we describe a unique cell type, entorhinal cortex (EC) path cells, the activity of which indicates whether the patient is taking a clockwise or counterclockwise path around the virtual square road. We find that many EC path cells exhibit this directional activity throughout the environment, in contrast to hippocampal neurons, which primarily encode information about specific locations. More broadly, these findings support the hypothesis that EC encodes general properties of the current context (e.g., location or direction) that are used by hippocampus to build unique representations reflecting combinations of these properties. | [[Publications#JacoEtal10|Jacobs et al. (2010b)]] examined recordings of single-neuron activity from neurosurgical patients playing a virtual-navigation video game. In addition to place cells, which encode the current virtual location, we describe a unique cell type, entorhinal cortex (EC) path cells, the activity of which indicates whether the patient is taking a clockwise or counterclockwise path around the virtual square road. We find that many EC path cells exhibit this directional activity throughout the environment, in contrast to hippocampal neurons, which primarily encode information about specific locations. More broadly, these findings support the hypothesis that EC encodes general properties of the current context (e.g., location or direction) that are used by hippocampus to build unique representations reflecting combinations of these properties. | ||
| + | |||
| + | '''Grid cells''' in the entorhinal cortex appear to represent spatial location via a triangular coordinate system. Such cells, which have been identified in rats, bats and monkeys, are believed to support a wide range of spatial behaviors. Recording neuronal activity from neurosurgical patients performing a virtual-navigation task, [[Publications#JacoEtal13|Jacobs et al. (2013)]] identified cells exhibiting grid-like spiking patterns in the human brain, suggesting that humans and simpler animals rely on homologous spatial-coding schemes. | ||
| + | |||
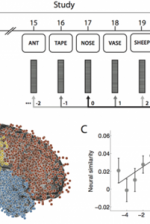
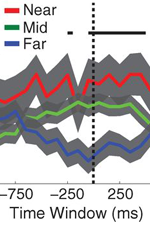
| + | Theories of episodic memory suggest that memory encoding and retrieval are facilitated by '''spatiotemporal context''': a continually updated representation of location in space and time. Recent work in the lab sought to examine the role of spatial context in human episodic memory retrieval through a hybrid spatial and episodic memory task. [[Publications#MillEtal13.pdf|Miller et al. (2013)]] identified place cells as neurosurgical patients performed a delivery task which required them to deliver items to different stores in a virtual town. Following this navigation task, patients were asked to freely recall the items they had delivered. Place cell activation patterns during the navigational task were then compared to the subsequent activation patterns that occurred during the free recall task. Miller et al. found that neural activity during the retrieval of each delivered item was similar to the neural activity associated with the location where that item was encoded. These findings demonstrate context-specific reinstatement of place-responsive cell activity at the time of recall, supporting theories that implicate contextual reinstatement as the basis for memory retrieval. | ||
{| style="margin: 0 auto;" cellpadding="20" | {| style="margin: 0 auto;" cellpadding="20" | ||
| − | + | [[File:MillerF2.png|center|thumb|225px|''Fig. 5:'' '''Place responsive cells.''' (a) Firing-rate maps for a cell responsive to northward traversals located in the hippocampus of one of the participants. (b) A cell responsive to eastward traversals recorded from a participant's entorhinal cortex. (c) Regional distribution of place-responsive cells across the entire data set of 371 single units (H, hippocampus; A, amygdala; EC, entorhinal cortex; PHG, parahippocampal gyrus; Ant, anterior medial temporal lobe).]] | |
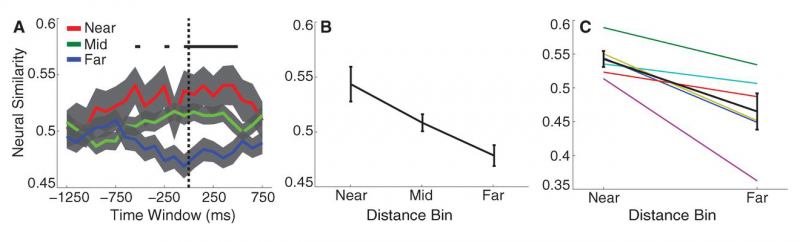
| − | + | |[[File:MillerF3.png|right|thumb|800px|''Fig. 6:'' '''Spatial context reinstatement.''' (a) Time courses of neural similarity between ensemble place-cell activity during navigation and during item recall for near, middle, and far spatial distance bins. Shaded regions indicate SEM across recalled items, the horizontal bars indicate statistically significant time points and the vertical dotted line indicates the onset of vocalization. (b) Average neural similarity for near, middle and far distance bins for the time period of -300 to 700ms relative to recall onset. Error bars indicate SEM across recalled items. (c) Neural similarity for near and far spatial distance bins for each participants (colored lines) and the participant average (black line) during -300 to 700ms relative to recall onset. Error bars indicate SEM across participants.]] | |
| − | + | ||
|} | |} | ||
| + | |||
| + | <!--{| style="margin: 0 auto;" cellpadding="20" | ||
| + | | [[File:grid_1.png|center|thumb|313px|''Fig. 8:'' '''Virtual navigation task.''' (a) Participant’s view of the experiment. (b) Mean duration of successive deliveries in the task, averaged across consecutive pairs of deliveries. (c) Mean excess path length. VRU is a measure of virtual distance. Error shading denotes 95% confidence intervals.]] || [[File:grid_2.png|center|thumb|399px|''Fig. 9:'' The activity of two example grid-like cells. Left, overhead view of the environment, with color representing the firing rate (in Hz) at each virtual location. Middle, two-dimensional autocorrelation of the cell’s activity. Peaks in the autocorrelation function determined the spacing and angle of the fitted c grid, which was then used to plot the estimated grid peaks (white ×) across the entire environment. Right, cell spike waveform; red denotes mean.]]--> | ||
| + | |||
| + | <!-- {| style="margin: 0 auto;" cellpadding="20" | ||
| + | | [[File:path_task.png|center|thumb|375px|''Fig. 8:'' '''The Yellow Cab virtual-navigation video game.''' (A) A patient’s on- screen view of the environment during the game. (B) Overhead map of the environment. Possible destination stores are brightly colored and outlined in red. Pale-colored buildings form the remainder of the outer and inner walls of the environment.]] || [[File:path_regions.png|center|thumb|230px|''Fig. 9:'' '''Regional distribution of path cells.''' Bars depict percentage of neurons observed in each brain area that were path cells. Dark shading indicates clockwise or counterclockwise path cells; light shading indicates complex path cells. Region key: A, amygdala; Cx, parietal and temporal cortices; EC, entorhinal cortex; Fr, Frontal cortices; H, hippocampus; PHG, parahippocampal gyrus.]] | ||
| + | |- | ||
| + | |colspan="2"| [[File:pathcell.png|center|thumb|650px|''Fig. 10:'' '''Clockwise and counterclockwise path cell activity.''' Firing rate of a clockwise path cell from a single patient's right entorhinal cortex during one testing session. Panel 1: Mean firing rate during clockwise movement at each location in the virtual environment. Color indicates the mean firing rate (in Hz), and gray lines indicate the path of the patient. Panel 2: Neuronal activity during counterclockwise movements. Panel 3: Indication of whether firing rate at each location was statistically greater (rank-sum test) during clockwise movements (red) or during counterclockwise movements (blue). Panel 4: Firing rate of this neuron combined across all regions of the environment for clockwise ("CW") and counterclockwise ("CCW") movements during straight movements and turns ("Turn"). Panel 4 inset: Activity of a neuron recorded from this same microelectrode in a different testing session; the two cells have very different firing rates and thus are likely distinct, nearby neurons. (Figure from Jacobs et al., 2010b.)]] | ||
| + | |} --> | ||
| + | |||
| + | <!--[[File:grid_cover.gif|thumb|250px|"Jacobs ''et al.'' show the first direct recordings of putative grid cells in the human entorhinal cortex during virtual navigation. Each of these cells supports spatial navigation by activating at a set of locations arranged in a triangular grid across an environment. Cover illustration by Brian Jacobs based on data from a human grid cell and a photograph by Joshua Jacobs." (''Nature'': [http://www.nature.com/neuro/journal/v16/n9/covers/index.html "About the cover"])]]--> | ||
| + | |||
| + | == Cognitive Neuromodulation == | ||
| + | |||
| + | The lab is also interested in how electrical stimulation can be used to therapeutically modulate memory function. The [[RAM|RAM]] project, supported by the Defense Advanced Research Projects Agency (DARPA), is a large-scale collaboration in which we use intracranial recording and stimulation to understand and affect memory function. By aggregating data collection from intracranial patients at many clinical centers, we are able to study in detail the neural mechanisms of memory encoding and retrieval across four tasks that address many core memory processes: free recall, categorized free recall, paired associate learning and spatial navigation. During performance of these tasks, we apply targeted electrical stimulation to specific nodes in the memory network, including the hippocampus, medial temporal lobe cortices and the frontal cortex. We then use multivariate machine learning methods to analyze the effects of stimulation on brain activity, and their relationship to memory performance. Our ultimate goal is to use this knowledge to understand how electrical stimulation can be used to treat memory dysfunction. | ||
| + | |||
| + | {| style="margin: 0 auto;" cellpadding="20" | ||
| + | <!--[[File:LabWiki_RAMFigure.png|center|thumb|800px|''Figure 7:'' '''Free recall and machine learning for cognitive neuromodulation.''' Left: an example free recall task used to collect record-only and stimulation data in intracranial patients. Right: we use machine learning methods, including classification of brain-wide spectral power, to understand how stimulation can evoke patterns of brain activity likely to lead to either remembering or forgetting.]]--> | ||
| + | |||
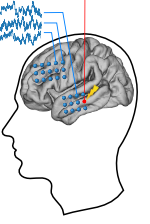
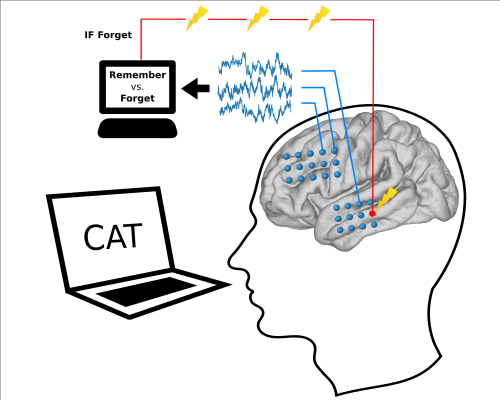
| + | [[File:closed_loop_stim.png|center|thumb|500px|''Figure 7:'' '''Closed-loop system for neuromodulation.''' Patients studied lists of words shown on a laptop screen while another computer monitored signals recorded from electrodes implanted directly in the brain. The computer analyzed the brain signals during learning to predict whether the patient would later remember the word. If the prediction was that the patient would forget the word, the computer triggered electrical stimulation to the lateral temporal lobe to improve learning.]] | ||
[[Category:public]] | [[Category:public]] | ||
Latest revision as of 16:19, 21 August 2023

|
|
|
|
|
Our lab investigates human memory and its neural basis using a combination of behavioral, computational, and neurophysiological methods. In our computational investigations, we build mathematical and computer-simulation models to account for the dynamics of memory retrieval in a variety of episodic and spatial memory tasks. Because behavioral data provides a sparse reflection of the brain’s activity supporting memory, we simultaneously record neurophysiological signals as patients with arrays of implanted electrodes perform memory tasks. In these investigations we study neural activity at multiple spatial scales, ranging from individual neurons to spatially-distributed networks of field-potential activity supporting memory. Several of our current projects also use electrical stimulation to manipulate memory circuits, both for understanding basic memory mechanisms and also for developing therapies to restore memory in patients with brain injury or neurological disease.
|
|
Computational models of human memory
To help explain the processes underlying encoding, organization and retrieval of episodic memories, we have developed, extended and refined a class of models based on the idea that items in memory become associated with a time-varying representation of spatio-temporal context. The temporal context model (TCM; Howard and Kahana, 2002 and TCM-A Sederberg, Howard, and Kahana, 2008) sought to explain the time-scale invariance of recency and contiguity effects in free recall, and dissociations between recall of recent and remote memories. Subsequent modeling work generalized TCM beyond temporal context to account for the influence of semantic knowledge on recall dynamics (CMR, Polyn, Norman, and Kahana (2009)). MATLAB scripts to run the CMR model can be downloaded here.
Lohnas, Polyn, and Kahana (2015) provided a major overhaul of the earlier CMR model, going beyond earlier modeling of individual lists to explain the interaction between memories learned across multiple experiences . In their CMR2 model, memory accumulates across multiple experimental lists, and temporal context is used both to focus retrieval on a target list and to censor retrieved information when its match to the current context indicates that it was learned in a non-target list. The model simultaneously accounts for a wide range of intralist and interlist phenomena, including the pattern of prior-list intrusions observed in free recall, build-up of and release from proactive interference, and the ability to selectively target retrieval of items on specific prior lists (Jang & Huber, 2008; Shiffrin, 1970). Healey and Kahana (2015) used CMR2 to better understand why memory tends to get worse as we age. By fitting CMR2 to the performance of individual younger and older adults, they identified deficits in four critical processes: sustaining attention across a study episode, generating retrieval cues, resolving competition, and screening for inaccurate memories (intrusions). Healey and Kahana also extended CMR2 to simulate a recognition memory task using the same mechanisms the free recall model uses to reject intrusions. Without fitting any additional parameters, the model accounts for age differences in recognition memory accuracy. Confirming a prediction of the model, free recall intrusion rates correlate positively with recognition false alarm rates. MATLAB scripts to run the CMR2 model can be downloaded here. Cohen and Kahana (2021, Psychological Review) introduced CMR3 to include the critical role of arousal and emotion in human memory. They applied their model to diverse phenomena including the role of emotion in organizing memories, state-dependent and mood congruent memory, the role of emotional experiences in producing persistent mood states, including depression, and a novel account of PTSD and its treatment.
A review of this line of research appeared in Kahana (2020), Computational Models of Memory Search, in the Annual Review of Psychology. Python code that runs CMR2 and CMR3 may be downloaded from the lab's publication page.
Neural and oscillatory correlates of episodic memory
We investigate the neurophysiology of episodic memory with electrocorticographic (ECoG) and single neuron recordings from neurosurgical patients who have had electrodes surgically implanted on the cortical surface of the brain or in the medial temporal lobes (including hippocampus) as part of the clinical process of localizing seizure foci. One focus of this research is to determine the oscillatory correlates of successful episodic memory formation and retrieval. Analyses of such recordings have shown that high-frequency activity (HFA, 70-150 Hz) increase while participants are studying words that they will successfully, as opposed to unsuccessfully, recall. HFA also rises just prior to successful vocal free recall (reviewed in Burke et al., 2015; for a video of the encoding effects click here here ) for a video of the retrieval effect click here here ).
|
Fig. 3: Free Recall Paradigm By using a free recall task in which participants study a list of words, we can measure episodic memory formation by comparing the spectral correlates associated with encoding items that are later recalled (red) or forgotten (blue). We record electroencephalographic (EEG) signals from subdurally implanted electrodes in patients with medically intractable epilepsy. We can extract spectral signals (power of a given frequency) from the raw EEG voltage traces for each item and measure when and where power at particular frequencies changes. Successful memory formation is associated with increases in HFA in left lateral temporal lobe, medial temporal lobe, and left prefrontal cortex. The same analyses can be performed on items during recall to assess when and where memories are retrieved. Successful memory retrieval is associated with increases in gamma band activity in the left neocortex and hippocampus as well as increases in theta band (4 -8 Hz) activity in right temporal lobe. |
The ability to reinstate this contextual information during memory search has been considered a hallmark of episodic, or event-based, memory. In Manning et al., 2011, we sought to determine whether contextual reinstatement may be observed in electrical signals recorded from the human brain during episodic recall. We examined ECoG activity from 69 neurosurgical patients as they studied and recalled lists of words in a delayed free recall paradigm (Fig. 4A), and computed similarity between the ECoG patterns recorded just prior to each recall with those recorded after the patient had studied each word. We found that, upon recalling a studied word, the recorded patterns of brain activity were not only similar to the patterns observed when the word was studied, but were also similar to the patterns observed during study of neighboring list words, with similarity decreasing reliably with positional distance (Fig. 4C), just as predicted by context reinstatement models of free recall. The degree to which individual patients exhibited this neural signature of contextual reinstatement was correlated with the contiguity effect as seen in Fig. 4D. In this way, the study provides neural evidence for contextual reinstatement in humans.
Human spatial memory and cognition
Our lab is also interested in the neural mechanisms underlying human spatial cognition. In this work, we use virtual reality computer games in which participants learn the locations of landmarks in virtual environments. To download a sample of a YellowCab session, click here. Using this approach, we have documented the existence and character of the 4-8 Hz theta rhythm in the human brain as participants learned to navigate through complex virtual environments (Kahana et al., 1999; Caplan et al., 2001; Caplan et al., 2003; Ekstrom et al., 2005; Jacobs et al., 2010a). Recording individual neurons during virtual navigation, we have discovered "place cells" in the human brain. These cells, which are found primarily in the human hippocampus, become active when a given spatial location is being traversed (Ekstrom et al., 2003). We also identified several other cellular responses during navigation: cells that become active in response to viewing a salient landmark (from any location), cells that become active when searching for a particular goal location (irrespective of location or view), and cells that respond when traveling in a given direction (bearing/heading).
Jacobs et al. (2010b) examined recordings of single-neuron activity from neurosurgical patients playing a virtual-navigation video game. In addition to place cells, which encode the current virtual location, we describe a unique cell type, entorhinal cortex (EC) path cells, the activity of which indicates whether the patient is taking a clockwise or counterclockwise path around the virtual square road. We find that many EC path cells exhibit this directional activity throughout the environment, in contrast to hippocampal neurons, which primarily encode information about specific locations. More broadly, these findings support the hypothesis that EC encodes general properties of the current context (e.g., location or direction) that are used by hippocampus to build unique representations reflecting combinations of these properties.
Grid cells in the entorhinal cortex appear to represent spatial location via a triangular coordinate system. Such cells, which have been identified in rats, bats and monkeys, are believed to support a wide range of spatial behaviors. Recording neuronal activity from neurosurgical patients performing a virtual-navigation task, Jacobs et al. (2013) identified cells exhibiting grid-like spiking patterns in the human brain, suggesting that humans and simpler animals rely on homologous spatial-coding schemes.
Theories of episodic memory suggest that memory encoding and retrieval are facilitated by spatiotemporal context: a continually updated representation of location in space and time. Recent work in the lab sought to examine the role of spatial context in human episodic memory retrieval through a hybrid spatial and episodic memory task. Miller et al. (2013) identified place cells as neurosurgical patients performed a delivery task which required them to deliver items to different stores in a virtual town. Following this navigation task, patients were asked to freely recall the items they had delivered. Place cell activation patterns during the navigational task were then compared to the subsequent activation patterns that occurred during the free recall task. Miller et al. found that neural activity during the retrieval of each delivered item was similar to the neural activity associated with the location where that item was encoded. These findings demonstrate context-specific reinstatement of place-responsive cell activity at the time of recall, supporting theories that implicate contextual reinstatement as the basis for memory retrieval.
Cognitive Neuromodulation
The lab is also interested in how electrical stimulation can be used to therapeutically modulate memory function. The RAM project, supported by the Defense Advanced Research Projects Agency (DARPA), is a large-scale collaboration in which we use intracranial recording and stimulation to understand and affect memory function. By aggregating data collection from intracranial patients at many clinical centers, we are able to study in detail the neural mechanisms of memory encoding and retrieval across four tasks that address many core memory processes: free recall, categorized free recall, paired associate learning and spatial navigation. During performance of these tasks, we apply targeted electrical stimulation to specific nodes in the memory network, including the hippocampus, medial temporal lobe cortices and the frontal cortex. We then use multivariate machine learning methods to analyze the effects of stimulation on brain activity, and their relationship to memory performance. Our ultimate goal is to use this knowledge to understand how electrical stimulation can be used to treat memory dysfunction.